International Certifications
International Clinical Evidence
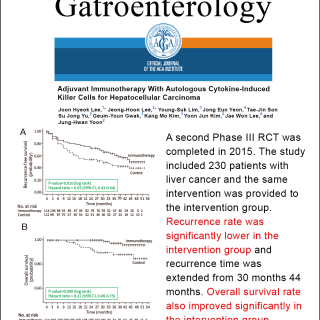
Source: Published in the Lancet international medical journal in 2000
🔼In 2000, the results of a Phase III clinical trial conducted in Japan were published in the internationally renowned medical journal, The Lancet. The trial involved 150 post-surgical liver cancer patients, with 76 patients randomly assigned to a treatment group receiving memory T cell therapy and 74 patients allocated to a control group. After a 7-year follow-up, the recurrence-free survival rate in the treatment group was significantly higher than in the control group (P<0.008). The trial demonstrated that a single treatment course could delay the recurrence time by up to 1.2 years. No severe side effects were found in the treatment group, with the main side effects being mild fever or local redness and swelling.
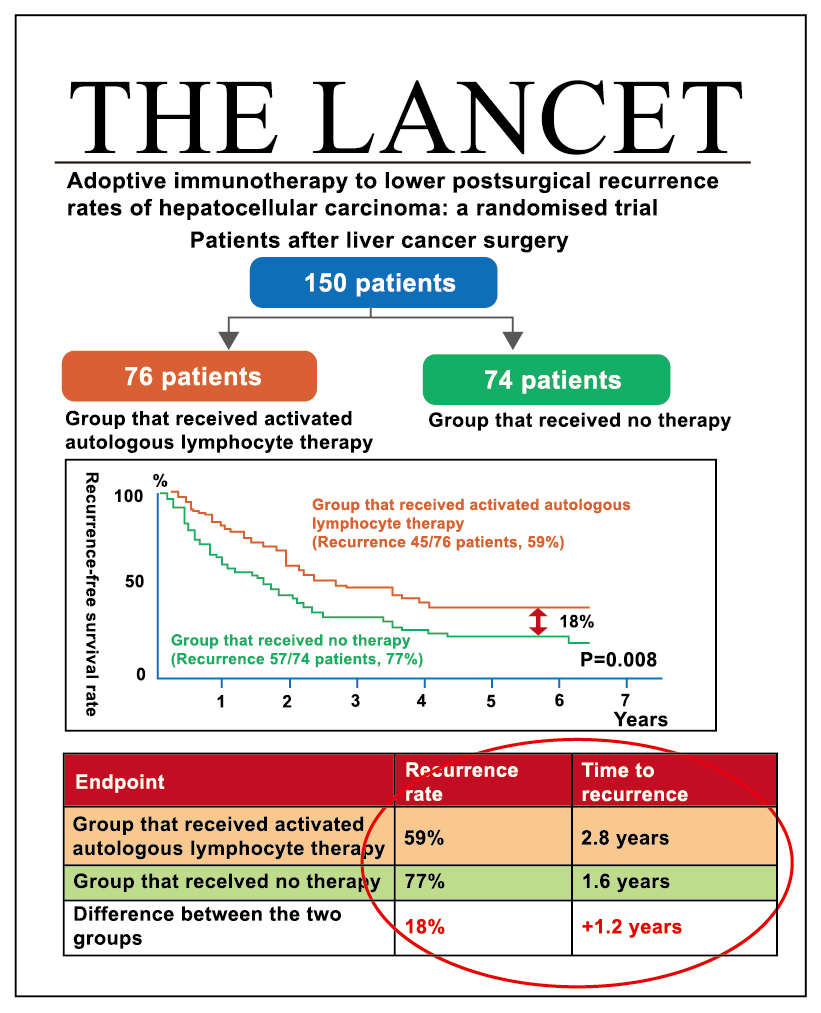
Source: 2015 Gastroenterology, an internationally renowned gastrointestinal medical journal
🔼The results of a Phase III clinical trial conducted by South Korea's GCC were published in the internationally renowned gastroenterology journal, Gastroenterology. The trial included 230 post-surgical liver cancer patients in stages I and II, who were randomly assigned to a treatment group receiving memory T cell therapy and a control group. After a 7-year follow-up, the recurrence-free survival rate in the treatment group was significantly higher than that in the control group (P=0.01). The overall survival rate in the treatment group was also superior to that in the control group (P=0.008). No severe side effects were found in the treatment group, with the main side effects being mild fever or local redness and swelling.
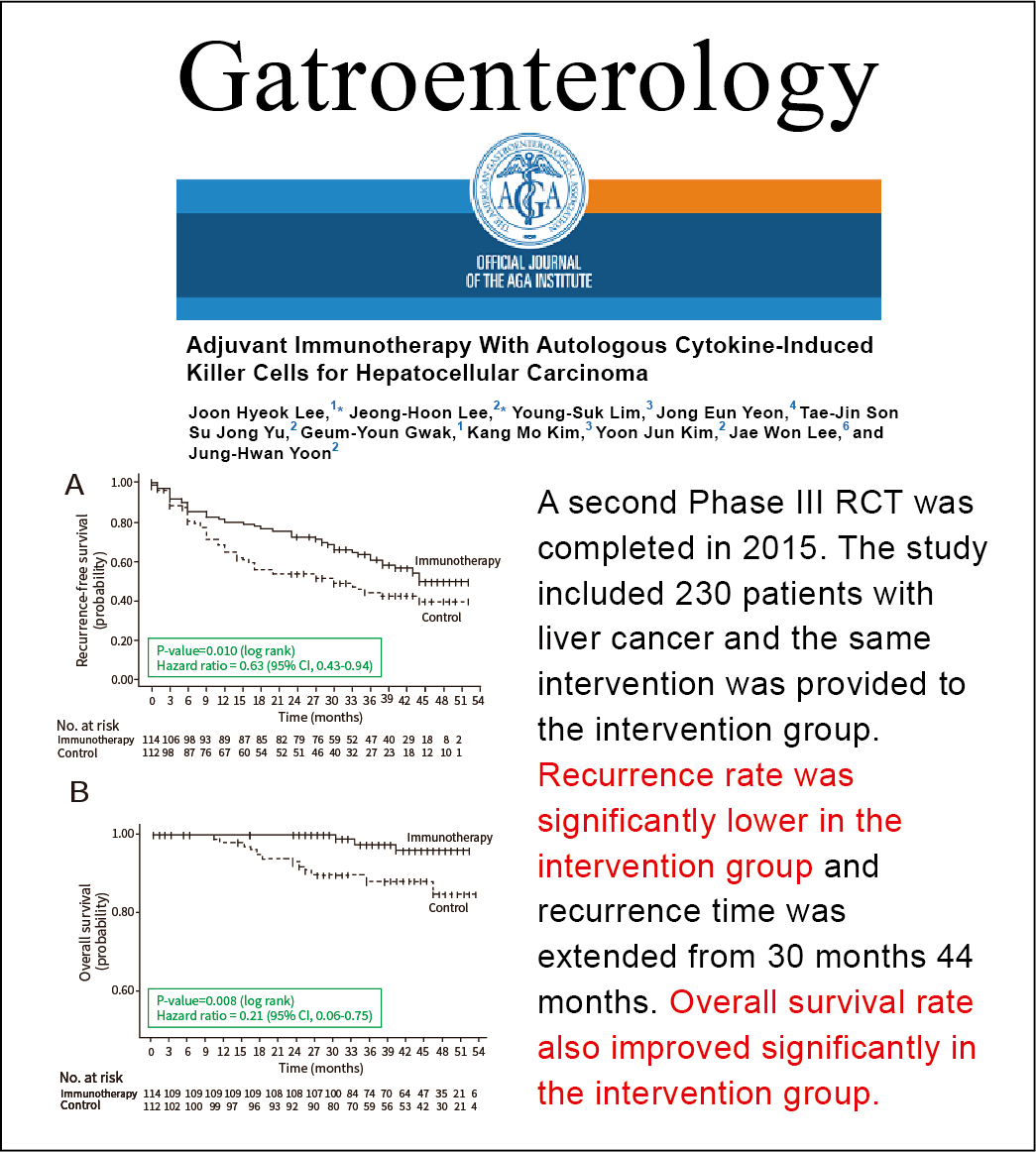
Source: 2019 Cancer Immunology, Immunotherapy International Medical Journal
🔼After completing the clinical trial, GCC obtained drug approval in South Korea and published the results of the Phase IV clinical trial in 2019. The results showed that, 90 months after a single treatment course, the recurrence-free survival rate, overall survival rate, and specific tumor survival rate in the treatment group remained superior to those in the control group (P=0.01, P=0.006, and P=0.02); the hazard ratio in the treatment group was 0.67, indicating a 33% reduction in the recurrence probability. This confirms that the technology can generate long-term memory in cancer patients and effectively prevent cancer recurrence.